Organocatalytic asymmetric Michael addition of 1-acetylcyclohexene and 1-acetylcyclopentene to nitroolefins†
Abstract
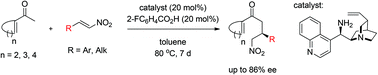
Enantioselective organocatalytic Michael addition reactions of 1-acetylcyclohexene, 1-acetylcyclopentene and 1-acetylcyclobutene to nitroolefins have been developed. This is the first report where an α-branched enone has been activated by an amine catalyst for the asymmetric Michael addition reaction to an electrophile. The Michael products have also been cyclized to bicyclic compounds.

 Please wait while we load your content...
Please wait while we load your content...