Enantioselective construction of a 2,2′-bisindolylmethane scaffold via catalytic asymmetric reactions of 2-indolylmethanols with 3-alkylindoles†
Abstract
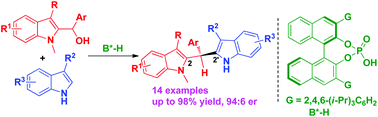
A chiral phosphoric acid-catalyzed asymmetric reaction of 2-indolylmethanols with 3-alkylindoles has been established, which constructed a biologically important 2,2′-bisindolylmethane scaffold in high yields and good enantioselectivities (up to 98% yield, 94 : 6 er). This protocol not only provides an efficient method for constructing a 2,2′-bisindolylmethane framework in an enantioselective form, but also promotes the development of 2-indolylmethanol-involved catalytic asymmetric transformations.

 Please wait while we load your content...
Please wait while we load your content...