Two-step one-pot synthesis of dihydropyrazinones as Xaa-Ser dipeptide isosteres through morpholine acetal rearrangement†
Abstract
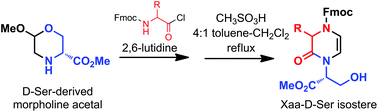
The synthesis of the uncommon dihydropyrazinone ring was accomplished by a two-step one pot process taking advantage of the ring rearrangement of N-acylated morpholine acetal derived from serine under acidic treatment in the presence of 2,6-lutidine. The mechanism involves an N-acyl iminium intermediate resulting from morpholine acetal ring opening, which occurs after a nucleophilic attack of the amino acid nitrogen atom to the acetal carbonyl atom. X-Ray diffraction analysis of the dihydropyrazinone, which may be exploited as a constrained Xaa-Ser dipeptide isostere, showed a planar assembly and the internal side-chain in axial orientation with respect to the cyclic molecular scaffold.

 Please wait while we load your content...
Please wait while we load your content...