Asymmetric synthesis of 3-substituted tetrahydro-2-benzazepines†
Abstract
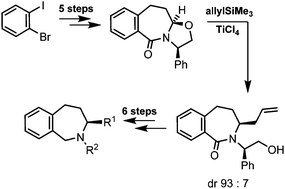
The enantiomerically and diastereomerically pure tricyclic oxazolidine cis-10 was prepared in a five step synthesis starting with 1-bromo-2-iodobenzene. Me3SiCN and allylSiMe3 reacted with cis-10 in the presence of TiCl4 to form the nitrile (3S)-11 and the allyl derivative (3S)-12 with high diastereoselectivity. The hydrogenolytic removal of the chiral auxiliary failed, since the endocyclic benzyl-N-bond was cleaved simultaneously. Therefore the N-(hydroxyethyl)amide of (3S)-12 was transformed into the enamide 27, which was hydrolyzed to afford the secondary amide 28. The enamide strategy to remove the chiral auxiliary from (3S)-11 led to complete racemization due to fast deprotonation in α-position of the cyano moiety. Two pairs of enantiomers 30a–b/ent-30a–b with prototypical σ substituents at the N-atom were prepared. The low σ1 affinity of the tetrahydro-2-benzazepines (ent-30b, Ki = 407 nM) is attributed to the short distance between the two lipophilic aromatic moieties.

 Please wait while we load your content...
Please wait while we load your content...