Decarboxylative functionalization of cinnamic acids
Abstract
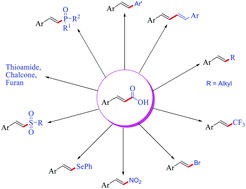
Decarboxylative functionalization of α,β-unsaturated carboxylic acids is an emerging area that has been developed significantly in recent years. This critical review focuses on the different decarboxylative functionalization reactions of cinnamic acids leading to the formation of various C–C and C–heteroatom bonds. Apart from metal carboxylates, decarboxylation in cinnamic acids has been achieved efficiently under metal-free conditions, particularly via the use of hypervalent iodine reagents. We believe this review will encourage organic chemists to develop vinylic decarboxylation in a more appealing way with an understanding of new mechanistic insight.

 Please wait while we load your content...
Please wait while we load your content...