Palladium-catalyzed direct C–H arylations of dioxythiophenes bearing reactive functional groups: a step-economical approach for functional π-conjugated oligoarenes†
Abstract
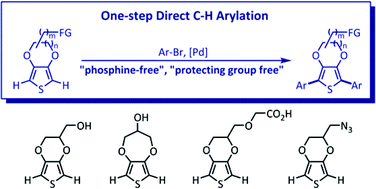
A Pd-catalyzed single-step C–H arylation of dioxythiophene derivatives bearing unprotected reactive functional groups (–OH, –COOH, –N3) in a phosphine-free manner has been developed. Various dioxythiopene-based oligoarenes with extended π-conjugation are obtained with good yields (up to 90%). These oligoarenes display suitable optical properties (absorption and emission maxima, quantum yields) and contain reactive functional groups suitable for further conjugations with bioactive molecules. This new methodology is step economical (fewer synthetic steps) and environmentally friendly (no toxic metal-containing by-products) and the oligoarenes synthesized are potentially applicable for bio-labeling, bioimaging, and biosensing.

 Please wait while we load your content...
Please wait while we load your content...