Discovery of novel isatin-based sulfonamides with potent and selective inhibition of the tumor-associated carbonic anhydrase isoforms IX and XII
Abstract
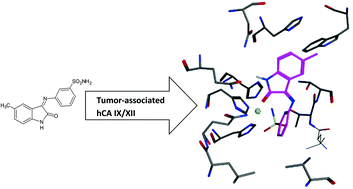
A series of 2/3/4-[(2-oxo-1,2-dihydro-3H-indol-3-ylidene)amino]benzenesulfonamides, obtained from substituted isatins and 2-, 3- or 4-aminobenzenesulfonamide, showed low nanomolar inhibitory activity against the tumor associated carbonic anhydrase (CA, EC 4.2.1.1) isoforms IX and XII – recently validated antitumor drug targets, being much less effective as inhibitors of the off-target cytosolic isoforms CA I and II.
- This article is part of the themed collection: Multivalent Biomolecular Recognition

 Please wait while we load your content...
Please wait while we load your content...