Copper catalysed direct amidation of methyl groups with N–H bonds†
Abstract
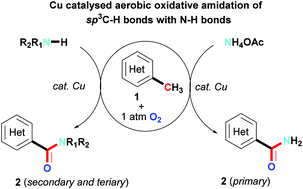
An efficient copper catalyzed direct aerobic oxidative amidation of methyl groups of azaarylmethanes with N–H bonds producing amides is successfully developed, which can produce primary, secondary and tertiary amides, including those with functional groups. This reaction represents a straightforward method for the preparation of amides from the readily available hydrocarbon starting materials.

 Please wait while we load your content...
Please wait while we load your content...