Fluorine containing amino acids: synthesis and peptide coupling of amino acids containing the all-cis tetrafluorocyclohexyl motif†
Abstract
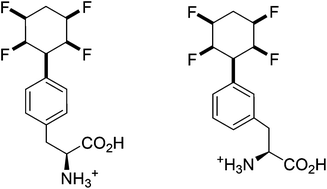
A synthesis of two (S)-phenylalanine derivatives is described which have the all-cis, 2,3,5,6-tetrafluorocyclohexyl motif attached to the aromatic ring at the meta and para positions; the para substituted isomer is elaborated into illustrative dipeptides via the free amine and carboxylate respectively demonstrating its utility as a novel amino acid for peptide synthesis and offering a vehicle for incorporation of this unique and facially polarized ring system into bioactive compounds.

 Please wait while we load your content...
Please wait while we load your content...