Cu(i)/TF-BiphamPhos-catalyzed asymmetric Michael addition of cyclic ketimino esters to alkylidene malonates†
Abstract
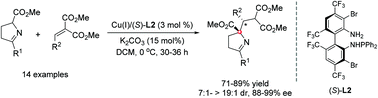
Cu(I)-catalyzed asymmetric Michael addition of cyclic ketimino esters with alkylidene malonates has been developed for efficient construction of β-branched α-amino acids containing adjacent quaternary and tertiary stereogenic centers in good yields with excellent diastereo-/enantioselectivities. The generated Michael adduct was further converted to the biologically important pyrrolizidine analogues via one-pot sequential reduction/lactamization.

 Please wait while we load your content...
Please wait while we load your content...