Divergent synthesis of 4,6-diarylated pyridin-2(1H)-ones from chalcones: novel access to 2,4,6-triaryl pyridines†
Abstract
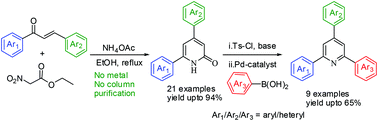
A wide range of 4,6-diarylated/heterylated pyridin-2(1H)-one derivatives were synthesized in good to excellent yields from 1,3-diarylated/heterylated-2-propen-1-ones (chalcones) in one pot under metal and base-free conditions. This domino reaction suggests a novel mechanism comprising of Michael addition followed by amination, subsequent intramolecular amidation and finally dehydronitrosation. The usefulness of the designed 4,6-diarylated/heterylated pyridin-2(1H)-one derivatives has further been demonstrated by synthesizing medicinally important 2,4,6-triaryl/heteryl pyridines via Pd-catalyzed cross-coupling reaction.

 Please wait while we load your content...
Please wait while we load your content...