Grignard-mediated reduction of 2,2,2-trichloro-1-arylethanones†
Abstract
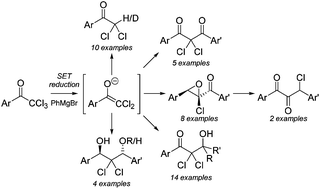
2,2,2-Trichloro-1-aryl-ethanones can be reduced by RMgX to the corresponding 2,2-dichloro-1-arylethen-1-olates and trapped with a range of electrophiles resulting in either reduction, reduction/aldol, reduction/Claisen condensation or reduction/aldol-Tishchenko products. In addition we demonstrate that 2,2-dichloro-1-arylethen-1-olates undergo counter-ion controlled Darzens condensations, which can be followed by a thermal rearrangement as a route to 1,3-diaryl-3-chloropropane-1,2-diones.

 Please wait while we load your content...
Please wait while we load your content...