CuX2-mediated oxybromination/aminochlorination of unsaturated amides: synthesis of iminolactones and lactams†
Abstract
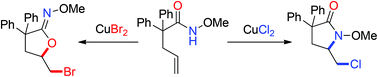
We report herein a CuX2-mediated halocyclization of γ,δ-unsaturated amides for the synthesis of functionalized iminolactones and lactams respectively under mild reaction conditions. Mechanism studies indicated that N-attack cyclization was via a radical route while oxycyclization was via a nucleophilic attack on the activated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond.
C bond.

 Please wait while we load your content...
Please wait while we load your content...