BINOL-Al catalysed asymmetric cyclization and amplification: preparation of optically active menthol analogs†
Abstract
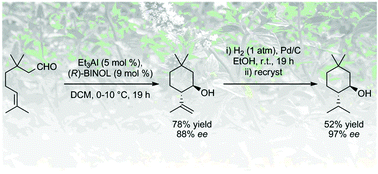
We report a highly selective asymmetric ring-closing ene reaction catalysed by aluminum complexes with chiral BINOL. This reaction yields optically active 6-membered cyclized alcohols from unsaturated aldehydes, with good diastereo- and enantioselectivities. Asymmetric amplification of this reaction was investigated by varying the ee of the BINOL employed in the catalyst.

 Please wait while we load your content...
Please wait while we load your content...