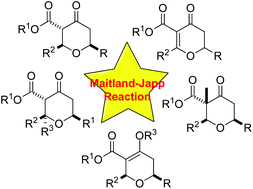
A Maitland–Japp inspired synthesis of dihydropyran-4-ones and their stereoselective conversion to functionalised tetrahydropyran-4-ones†
Abstract
The Maitland–Japp reaction has been extended to the synthesis of highly functionalised dihydropyran-4-ones. These dihydropyran-4-ones can in turn be converted stereoselectively into tetrahydropyran-4-ones with tertiary and quaternary stereocentres via the one-pot addition of hydride or carbon nucleophiles and trapping with carbon electrophiles. The utility of this method is demonstrated by providing access to the functionalised tetrahydropyran units present in a component of the Civet fragrance and the anticancer polyketide lasonolide A.


 Please wait while we load your content...
Please wait while we load your content...