Intramolecular direct aldol reactions of sugar 2,7-diketones: syntheses of hydroxylated cycloalka(e)nones†
Abstract
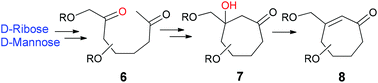
A regio- and stereoselective intramolecular direct aldol reaction of 2,7-diketones derived from carbohydrates has been developed to construct cycloalkanones 7, which were dehydrated to obtain heavily oxygenated cycloalkenones 8.

 Please wait while we load your content...
Please wait while we load your content...