Synthesis and photosensitivity of isoxazolin-5-one glycosides†
Abstract
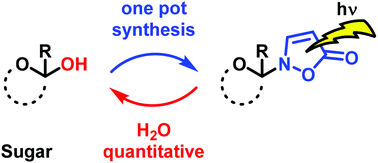
A novel procedure for the synthesis of isoxazolin-5-one glycosides starting from unprotected carbohydrates is described. The substrate scope of the one-pot synthetic protocol was explored using D-configured glucose, xylose, maltose, fructose, ribose and 2-deoxyribose. Naturally occurring 2-(β-D-glucopyranosyl)-3-isoxazolin-5-one and four novel isoxazolin-5-one glycosides derived from xylose, maltose and fructose were synthesized and purified by flash chromatography. The compounds were characterized in terms of chemical structure, photophysical properties as well as pH stability. The photohydrolysis rates of the synthesized glycosides were compared with uridine as a standard to determine the quantum yields for the photoreactions in water.

 Please wait while we load your content...
Please wait while we load your content...