Efficient access to enantiopure 1,3-disubstituted isoindolines from selective catalytic fragmentation of an original desymmetrized rigid overbred template†
Abstract
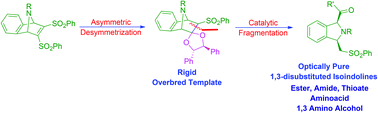
An efficient and scalable synthesis of various enantiopure 1,3- disubstituted isoindolines is reported. The base catalyzed nucleophilic fragmentation of a rigid overbred template is established with various substrates to afford the corresponding 1,3-disubstituted isoindoline ester, amide, thioate, 1,3-amino alcohol and isoindolylcarboxylic acid. The crucial rigid overbred template is synthesized in an optically pure form in multigram scale by asymmetric desymmetrization of the corresponding meso compound.

 Please wait while we load your content...
Please wait while we load your content...