Novel carbapenem chalcone derivatives: synthesis, cytotoxicity and molecular docking studies†
Abstract
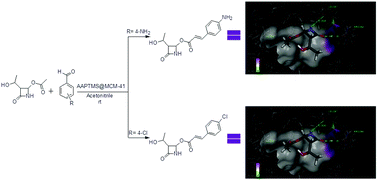
A one-pot efficient synthetic protocol is described for the synthesis of carbapenem chalcone derivatives using AAPTMS@MCM-41 heterogeneous catalyst. Various substituted aromatic aldehydes were attached to highly chiral and reactive carbapenem using this approach. The cytotoxic activity evaluation of all synthesized compounds was performed against lung cancer cell lines (A-549) and breast cancer cell lines (MCF-7) using the MTT assay. Among the tested compounds, compound CPC-2 showed better activity against MCF-7 cell lines with an IC50 value 2.52 μM mL−1; whereas compound CPC-4 showed good activity against A-549 cell lines with an IC50 value 1.59 μM mL−1. In order to support the observed activity profiles, the representative compounds were flexibly docked into the active sites of the Anaplastic Lymphoma Kinase (ALK) enzyme and the Estrogen receptor (ERβ). The most active anticancer compounds exhibited stronger binding affinities for proteins.

 Please wait while we load your content...
Please wait while we load your content...