Nickel-catalyzed synthesis of diarylsulfides and sulfones via C–H bond functionalization of arylamides†
Abstract
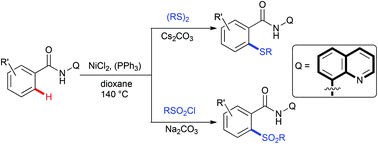
The direct sulfenylation and sulfonylation of (sp2)C–H bonds of benzamide derivatives were achieved using a Ni catalyst with the aid of an 8-aminoquinoline moiety as a bidentate directing group. These protocols represent a convenient route for the formation of valuable diaryl sulfides and sulfones in moderate to excellent yields.

 Please wait while we load your content...
Please wait while we load your content...