Pd(ii)-catalyzed C(sp3)–H arylation of amino acid derivatives with click-triazoles as removable directing groups†
Abstract
By using click-triazoles as conveniently approachable and removable directing groups, the direct palladium-catalyzed C(sp3)–H arylation of amino acid derivatives with various aryl iodides bearing different electronic properties has been achieved. Notably, the desired amino acid molecule can be obtained by the cleavage of the tethered click-triazoles after the catalytic reaction, which aims to provide a practical protocol for the accessibility of both natural and synthetic amino acids.
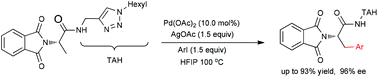

 Please wait while we load your content...
Please wait while we load your content...