Buffer catalyzed cleavage of uridylyl-3′,5′-uridine in aqueous DMSO: comparison to its activated analog, 2-hydroxypropyl 4-nitrophenyl phosphate†
Abstract
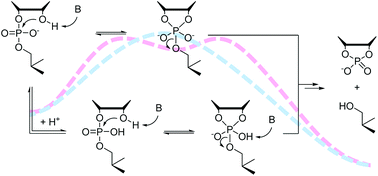
Buffer catalysis of the cleavage and isomerization of uridylyl-3′,5′-uridine (UpU) has been studied over a wide pH range in 80% aq. DMSO. The diminished hydroxide ion concentration in this solvent system made catalysis by amine buffers (morpholine, 4-hydroxypiperidine and piperidine) visible even at relatively low buffer concentrations (10–200 mmol L−1). The observed catalysis was, however, much weaker than what has been previously reported for the activated RNA model 2-hydroxypropyl 4-nitrophenyl phosphate (HPNP) in the same solvent system. In the case of morpholine, contribution of both the acidic and the basic buffer constituent was significant, whereas with 4-hydroxypiperidine and piperidine participation of the acidic constituent could not be established unambiguously. The results underline the importance of using realistic model compounds, along with activated ones, in the study of the general acid/base catalysis of RNA cleavage.


 Please wait while we load your content...
Please wait while we load your content...