Conformationally restricted 3′-modified ABA analogs for controlling ABA receptors†
Abstract
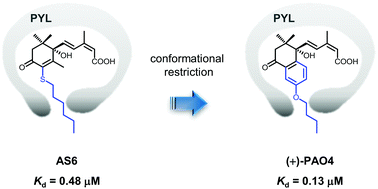
The physiological functions of abscisic acid (ABA) are regulated by a signal transduction pathway involving cytosolic ABA receptors, which include 14 PYR/PYL/RCAR (PYL) proteins in Arabidopsis. The development of a PYL antagonist could be a valuable tool to improve our understanding of the roles of ABA. We previously developed 3′-hexylsulfanyl-ABA (AS6), whose S-hexyl chain blocks protein phosphatase 2C (PP2C) binding by steric hindrance. This finding not only validated our structure-based approach to the design of a PYL antagonist, but also provided a basis for the development of a more potent or subclass/subtype selective PYL antagonist. In the present study, we synthesized a conformationally restricted analog of AS6, namely propenyl-ABA with an O-butyl chain (PAO4), to improve the affinity for PYL proteins by reducing the entropic penalty for binding to the receptors. In seed germination assays, (+)-PAO4 was a slightly stronger antagonist than AS6 in Arabidopsis and a significantly stronger antagonist in lettuce. Analysis of the thermodynamic parameters associated with the formation of the Arabidopsis PYL–(+)-PAO4 complex revealed that (+)-PAO4 binds more strongly to PYL5 than AS6 owing to an entropic advantage. In PP2C assays, this enhancement effect was observed only for the monomeric PYL subclass containing PYL5, suggesting that (+)-PAO4 is more effective than AS6 in physiological events involving monomeric PYL proteins as ABA receptors.

 Please wait while we load your content...
Please wait while we load your content...