Hemisynthesis of deuteriated adenosylhopane and conversion into bacteriohopanetetrol by a cell-free system from Methylobacterium organophilum†
Abstract
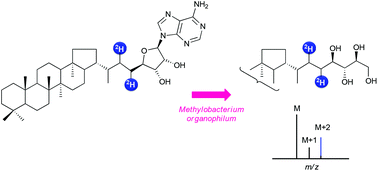
Adenosylhopane is a putative precursor of the widespread bacterial C35 biohopanoids. A concise and flexible hemisynthesis of adenosylhopane has been developed including as key steps a cross metathesis between two olefins containing either the hopane moiety or a protected adenosine derivative and a subsequent diimide reduction of the resulting olefin. Reduction by deuteriated diimide allowed deuterium labelling. This synthetic protocol represents a versatile tool to access to deuteriated composite bacterial hopanoids required for biosynthetic studies. Deuteriated adenosylhopane was thus converted into bacteriohopanetetrol by a crude cell-free system from Methylobacterium organophilum in the presence of NADPH, showing for the first time the precursor to product relationship between these two bacterial metabolites.

 Please wait while we load your content...
Please wait while we load your content...