An asymmetric pericyclic cascade approach to 3-alkyl-3-aryloxindoles: generality, applications and mechanistic investigations†
Abstract
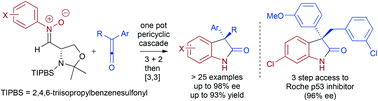
The reaction of L-serine derived N-arylnitrones with alkylarylketenes generates asymmetric 3-alkyl-3-aryloxindoles in good to excellent yields (up to 93%) and excellent enantioselectivity (up to 98% ee) via a pericyclic cascade process. The optimization, scope and applications of this transformation are reported, alongside further synthetic and computational investigations. The preparation of the enantiomer of a Roche anti-cancer agent (RO4999200) 1 (96% ee) in three steps demonstrates the potential utility of this methodology.

 Please wait while we load your content...
Please wait while we load your content...