Diastereoselective desymmetrization of diarylphosphinous acid-borane amides under Birch reduction†
Abstract
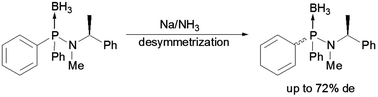
Treatment of diarylphosphinous acid-borane amides possessing chiral amido functionality with an alkali metal solution in liquid ammonia induced a preferential dearomatization of one aryl substituent at phosphorus leading to the formation of non-equimolar amounts of diastereomers. Diastereoselectivity of dearomatization depends strongly on the structure of a chiral auxiliary.

 Please wait while we load your content...
Please wait while we load your content...