Synthetic and immunological studies of N-acyl modified S-linked STn derivatives as anticancer vaccine candidates†
Abstract
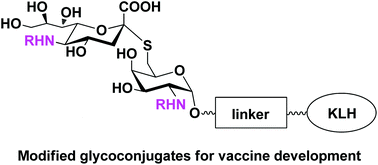
It is well known that tumor cells express some aberrant glycans, termed tumor-associated carbohydrate antigens (TACAs). TACAs are good targets for the development of carbohydrate-based anticancer vaccines. However, one of the major problems is that carbohydrate antigens possess a weak immunogenicity. To tackle this problem, a number of unnatural N-modified S-linked STn analogues were designed and prepared. Reaction of the modified STn disaccharides with bifunctional adipic acid p-nitrophenyl diester provided the corresponding activated esters, which was followed by the conjugation with keyhole limpet hemocyanin (KLH), affording the corresponding protein conjugates. The immunological properties of these glycoconjugates were evaluated in a mouse model. The results showed that the modified glycoconjugates stimulated the production of IgG antibodies that are capable of recognizing the naturally occurring STn antigen, helping the discovery of carbohydrate-based anticancer vaccine candidates.

 Please wait while we load your content...
Please wait while we load your content...