Consecutive three-component synthesis of (hetero)arylated propargyl amides by chemoenzymatic aminolysis–Sonogashira coupling sequence†
Abstract
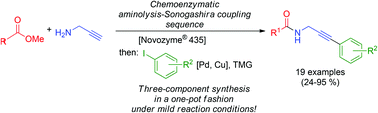
A novel chemoenzymatic three-component synthesis of (hetero)arylated propargyl amides in good yields based upon Novozyme® 435 (Candida antarctica lipase B (CAL-B)) catalyzed aminolysis of methyl carboxylates followed by Sonogashira coupling with (hetero)aryliodides in a consecutive one-pot fashion has been presented. This efficient methodology can be readily concatenated with a CuAAC (Cu catalyzed alkyne azide cycloaddition) as a third consecutive step to furnish 1,4-disubstituted 1,2,3-triazole ligated arylated propargyl amides. This one-pot process can be regarded as a transition metal catalyzed sequence that takes advantage of the copper source still present from the cross-coupling step.

 Please wait while we load your content...
Please wait while we load your content...