Photocontrol of ion permeation in lipid vesicles with amphiphilic dithienylethenes†
Abstract
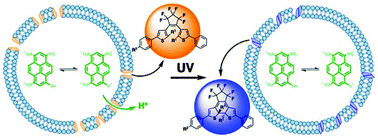
The integration of photochromic dithienylethenes (DTEs) with lipid vesicles as photoresponsive membrane disruptors for ion transport applications has been examined. We have synthesized three amphiphilic DTEs 1–3 that incorporate a terminally charged alkyl chain, and contain methyl or phenylethynyl substituents at the reactive carbons. Our photochromic reactivity studies suggest that the inclusion of a single alkyl chain favors the photoactive antiparallel conformation of DTEs, given the significant improvement in the cyclization quantum yield over previous phenylethynyl derivatives. Our ion permeation studies show that the open-ring isomers of these DTEs are more disruptive than the closed-ring isomers in the four lipid vesicle systems studied, regardless of their lamellar phase at room temperature. In addition, a steric effect was clearly observed as DTEs incorporating the comparatively smaller methyl group exhibited lower rates of ion permeation than the bulkier phenylethynyl group. In all cases, UV irradiation led to a reduction in ion permeability. In fact, the methyl analog exhibited a significant reduction in ion permeability in gel-phase lipid vesicles upon UV exposure. Also, the hexyl chain derivatives had a greater effect on membrane permeability than the dodecyl derivative owing to their relative position in the bilayer membrane of lipid vesicles.

 Please wait while we load your content...
Please wait while we load your content...