Enhancing the anti-inflammatory activity of chalcones by tuning the Michael acceptor site†
Abstract
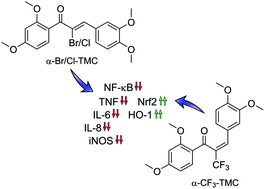
Inflammatory signaling pathways orchestrate the cellular response to infection and injury. These pathways are known to be modulated by compounds that alkylate cysteinyl thiols. One class of phytochemicals with strong thiol alkylating activity is the chalcones. In this study we tested fourteen chalcone derivatives, α-X-substituted 2′,3,4,4′-tetramethoxychalcones (α-X-TMCs, X = H, F, Cl, Br, I, CN, Me, p-NO2-C6H4, Ph, p-OMe-C6H4, NO2, CF3, COOEt, COOH), for their ability to modulate inflammatory responses, as monitored by their influence on heme oxygenase-1 (HO-1) activity, inducible nitric oxide synthase (iNOS) activity, and cytokine expression levels. We confirmed that the transcriptional activity of Nrf2 was activated by α-X-TMCs while for NF-κB it was inhibited. For most α-X-TMCs, anti-inflammatory activity was positively correlated with thiol alkylating activity, i.e. stronger electrophiles (X = CF3, Br and Cl) being more potent. Notably, this correlation did not hold true for the strongest electrophiles (X = CN and NO2) which were found to be ineffective as anti-inflammatory compounds. These results emphasize the idea that chemical fine-tuning of electrophilicity is needed to achieve and optimize desired therapeutic effects.

 Please wait while we load your content...
Please wait while we load your content...