A unified lead-oriented synthesis of over fifty molecular scaffolds†
Abstract
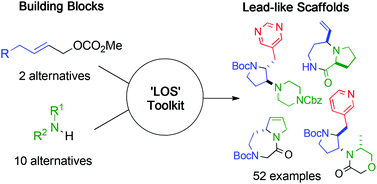
Controlling the properties of lead molecules is critical in drug discovery, but sourcing large numbers of lead-like compounds for screening collections is a major challenge. A unified synthetic approach is described that enabled the synthesis of 52 diverse lead-like molecular scaffolds from a minimal set of 13 precursors. The divergent approach exploited a suite of robust, functional group-tolerant transformations. Crucially, after derivatisation, these scaffolds would target significant lead-like chemical space, and complement commercially-available compounds.
 Please wait while we load your content...
Please wait while we load your content...