NBD-based fluorescent chemosensor for the selective quantification of copper and sulfide in an aqueous solution and living cells†
Abstract
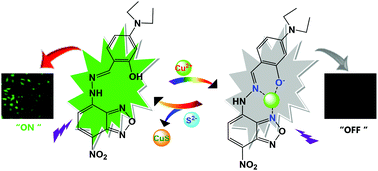
Chemosensors play important roles in cation and anion recognition in biological, industrial, and environmental processes. Although many efforts have been made to develop artificial fluorescent receptors for Cu2+ and S2−, their applications in the detection in bulk solutions are limited. In this work, we report a novel fluorescence chemosensor (NL) based on the 7-nitrobenz-2-oxa-1,3-diazole (NBD) fluorophore for the quantification of Cu2+ and S2− in single intact cells. NL specifically binds to Cu2+ in the presence of other competing cations, and evident changes in UV-vis and fluorescence spectra in HEPES buffer are noticed. Based on the displacement approach, the selective sense S2− with the in situ generated NL-Cu2+ ensemble gives a remarkable recovery of fluorescence and UV-vis absorption spectra. The detection limits of NL for Cu2+ and NL-Cu2+ for S2− were estimated to be 1.6 nM and 0.17 μM, respectively. NL and the resultant complex NL-Cu2+ exhibit low cytotoxicity and cell-membrane permeability, which makes them capable of Cu2+ and S2− imaging and quantification in living MDA-MB-231 cells.

 Please wait while we load your content...
Please wait while we load your content...