Transition metal catalyzed meta-C–H functionalization of aromatic compounds
Abstract
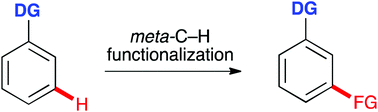
Direct functionalization of C–H bonds represents a powerful strategy for the synthesis of complex organic compounds due to its inherent efficiency. Among various approaches, transition metal catalyzed direct activation of unreactive C–H bonds is particularly effective for this purpose. However, the development of practical methods for transition metal catalyzed direct C–H functionalization has been challenging. Apart from identifying the reaction conditions that allow the activation of relatively unreactive C–H bonds, these reactions need to be selective, allowing one C–H bond to be differentiated from the rest of the ubiquitous C–H bonds of the compound. Whereas directing group guided, transition metal catalyzed ortho-C–H functionalization of aromatic compounds has seen significant growth in the past few decades, methods for meta-C–H functionalization of arenes have also emerged. This review summarizes approaches for directing group guided, transition metal catalyzed meta-C–H functionalization of aromatic compounds. Some steric-controlled, transition metal catalyzed formal meta-C–H functionalization reactions without coordinating directing groups are also discussed.
 Please wait while we load your content...
Please wait while we load your content...