Synthesis and tautomerization of hydroxylated isoflavones bearing heterocyclic hemi-aminals†
Abstract
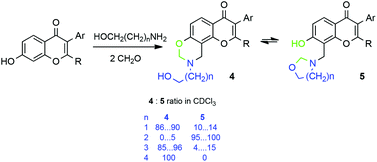
The aminomethylation of hydroxylated isoflavones with 2-aminoethanol, 3-amino-1-propanol, 4-amino-1-butanol, and 5-amino-1-pentanol in the presence of excess formaldehyde led principally to 9-(2-hydroalkyl)-9,10-dihydro-4H,8H-chromeno[8,7-e][1,3]-oxazin-4-ones 4 and/or the tautomeric 7-hydroxy-8-(1,3-oxazepan-3-ylmethyl)-4H-chromen-4-ones 5. The ratio of these tautomers was dependent on solvent polarity, electronic effects of aryl substituents in the isoflavone and the structure of the amino alcohol. NMR studies confirmed the interconversion of tautomeric forms.

 Please wait while we load your content...
Please wait while we load your content...