Modulation of the charge transfer and photophysical properties in non-fused tetrathiafulvalene-benzothiadiazole derivatives†
Abstract
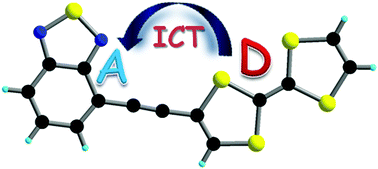
Bis(thiomethyl)- and bis(thiohexyl)-tetrathiafulvalene-bromo-benzothiadiazoles, containing electron donor tetrathiafulvalene (TTF) and electron acceptor benzothiadiazole (BTD) units, have been prepared by Stille coupling reactions between the TTF-SnMe3 precursors and BTD-Br2. In another series of experiments, TTF-acetylene-BTD compounds have been synthesized by Sonogashira coupling between either TTF-acetylenes and BTD-Br2 in low yields, or TTF-iodine and BTD-acetylene in moderate yields. In the compound TTF-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C-BTD the TTF and BTD units are coplanar in the solid state, as shown by the single crystal X-ray structure, and there is segregation in the packing between the donor and acceptor units. All the derivatives have good electron donor properties, as determined by cyclic voltammetry measurements, and they can also be reversibly reduced thanks to the presence of the BTD moiety. UV-visible spectroscopy and photophysical investigations show the presence of an intramolecular charge transfer (ICT) band and an emission band originating from the charge transfer. Both the absorption and the emission are modulated by the substitution scheme and the insertion of the acetylenic bridge.
C-BTD the TTF and BTD units are coplanar in the solid state, as shown by the single crystal X-ray structure, and there is segregation in the packing between the donor and acceptor units. All the derivatives have good electron donor properties, as determined by cyclic voltammetry measurements, and they can also be reversibly reduced thanks to the presence of the BTD moiety. UV-visible spectroscopy and photophysical investigations show the presence of an intramolecular charge transfer (ICT) band and an emission band originating from the charge transfer. Both the absorption and the emission are modulated by the substitution scheme and the insertion of the acetylenic bridge.

 Please wait while we load your content...
Please wait while we load your content...