Synthesis of maculalactone A and derivatives for environmental fate tracking studies†
Abstract
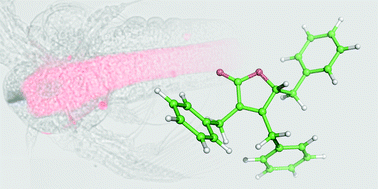
Maculalactone A (1) constitutes a promising antifouling agent, inhibiting the formation of biofilms in marine and freshwater systems. In this study, we developed a new route, based on a late-stage formation of the butenolide core, leading to the total synthesis of maculalactone A (three steps, overall yield of 45%) and delivering material on a gram scale. In addition, analogues of the title compound were assayed concerning their biological activity, utilizing Artemia franciscana and Thamnocephalus platyurus. The most active analogue was functionalized with a rhodamine B fluorophore and was utilized in an in vivo staining experiment in Artemia salina. Two different tissues were found to accumulate this maculalactone A derivative.

 Please wait while we load your content...
Please wait while we load your content...