The development of a complementary pathway for the synthesis of aliskiren†
Abstract
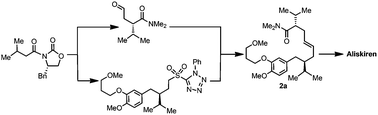
The synthesis of aliskiren (1), a recently marketed drug for the treatment of hypertension, is presented. The focus of our synthetic effort is to develop an efficient pathway for the synthesis of (2S,7R,E)-2-isopropyl-7-(4-methoxy-3-(3-methoxypropoxy) benzyl)-N,N,8-trimethylnon-4-enamide (2a), which has been used as the advanced intermediate toward aliskiren. After an extensive investigation of three different strategies designed to construct the E-olefin functionality in 2a by employing the olefin cross-metathesis, Horner–Wadsworth–Emmons (HWE), and Julia-type olefinations, we have established a new protocol for the synthesis of 2a with a substantially improved overall efficiency in terms of the yield (ca. 33%), and diastereo- and E/Z-selectivity. The key transformations were the Evans chiral auxiliary-aided asymmetric allylation for the synthesis of the appropriate chiral intermediates in excellent enantiomeric purity of higher than 97% ee and a modified Julia–Kocienski olefination for the highly selective construction of E-2a with up to 13.6 : 1 E/Z ratio from the chiral intermediates. Consequently, the results provide an appealing option for the synthesis of aliskiren.

 Please wait while we load your content...
Please wait while we load your content...