Palladium-catalyzed oxidative cross-coupling of azole-4-carboxylates with indoles: an approach to the synthesis of pimprinine†
Abstract
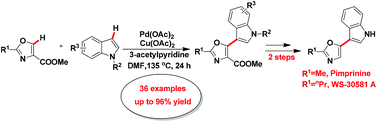
An efficient and straightforward method for the production of 5-(3-indolyl)azoles incorporating the privileged structures indoles and azoles via palladium-catalyzed double C–H bond cleavage under mild conditions was disclosed. As expected, this protocol provided an easy method for the synthesis of indole alkaloids pimprinine and WS-30581 A in moderate yields.

 Please wait while we load your content...
Please wait while we load your content...