Preparation of cycloheptane ring by nucleophilic cyclopropanation of 1,2-diketones with bis(iodozincio)methane†
Abstract
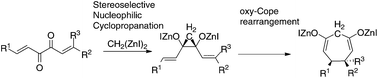
The nucleophilic cyclopropanation of hexa-1,5-diene-3,4-diones with bis(iodozincio)methane afforded the Zn alkoxides of cis-dialkenylcyclopropane-1,2-diols stereoselectively. The subsequent oxy-Cope rearrangement afforded the corresponding Zn alkoxides of 5,6-dialkylcyclohepta-3,7-diene-1,3-diols.

 Please wait while we load your content...
Please wait while we load your content...