Antisense precision polymer micelles require less poly(ethylenimine) for efficient gene knockdown†
Abstract
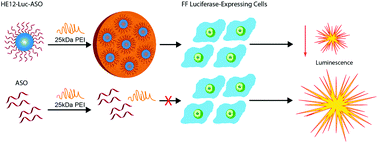
Therapeutic nucleic acids are powerful molecules for shutting down protein expression. However, their cellular uptake is poor and requires transport vectors, such as cationic polymers. Of these, poly(ethylenimine) (PEI) has been shown to be an efficient vehicle for nucleic acid transport into cells. However, cytotoxicity has been a major hurdle in the development of PEI–DNA complexes as clinically viable therapeutics. We have synthesized antisense–polymer conjugates, where the polymeric block is completely monodisperse and sequence-controlled. Depending on the polymer sequence, these can self-assemble to produce micelles of very low polydispersity. The introduction of linear poly(ethylenimine) to these micelles leads to aggregation into size-defined PEI-mediated superstructures. Subsequently, both cellular uptake and gene silencing are greatly enhanced over extended periods compared to antisense alone, while at the same time cellular cytotoxicity remains very low. In contrast, gene silencing is not enhanced with antisense polymer conjugates that are not able to self-assemble into micelles. Thus, using antisense precision micelles, we are able to achieve significant transfection and knockdown with minimal cytotoxicity at much lower concentrations of linear PEI then previously reported. Consequently, a conceptual solution to the problem of antisense or siRNA delivery is to self-assemble these molecules into ‘gene-like’ micelles with high local charge and increased stability, thus reducing the amount of transfection agent needed for effective gene silencing.

 Please wait while we load your content...
Please wait while we load your content...