A cationic azobenzene-surfactant-modified graphene hybrid: unique photoresponse and electrochemical behavior†
Abstract
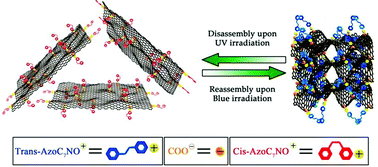
Surfactant-modified graphene hybrids containing azobenzene groups were for the first time prepared, and the electrochemical performance was investigated. The hybrids were obtained by electrostatic interactions between cationic azobenzene-surfactants and negatively charged graphene oxide in water. The electrostatic interactions, chemical structure and photoresponse of the hybrids were measured by using zeta potential values, fluorescence spectra, FTIR, XPS, XRD, SEM, UV-Vis absorption, AFM and Raman spectra. The electrochemical performance was estimated using cyclic voltammetry. The results show that strong electrostatic interactions exist between the azobenzene surfactants and graphene oxide. Notably, this azobenzene–graphene hybrid can self-assemble into aggregation structures in aqueous solution. Besides, the self-assembly can be reversibly controlled by ultraviolet light (365 nm) and blue light (455 nm) irradiation. This process is driven by the photoinduced polarity change of the cationic azobenzene surfactant and is responsible for the graphene hybrids’ electrochemical performance. It is the first example of the reversible self-assembly of graphene driven by light irradiation.

 Please wait while we load your content...
Please wait while we load your content...