Hydrophilic and blue fluorescent N-doped carbon dots from tartaric acid and various alkylol amines under microwave irradiation†
Abstract
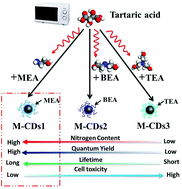
The desired control of particle size, doping element composition, and surface structure of carbon dots (CDs) are vital for understanding the fluorescence mechanism and exploring their potential applications. Herein, nitrogen-doped CDs (N-doped CDs) have been synthesized with tartaric acid and various alkylol amines (monoethanolamine, biethanolamine and triethanolamine) under microwave irradiation. A systematic investigation was performed to characterize the N-doped CDs. It is found that with increasing nitrogen proportion, the fluorescent quantum yield and lifetime of N-doped CDs increases, whereas cell toxicity decreases. In other words, N-doped CDs synthesized by tartaric acid and monoethanolamine have the highest nitrogen content, the highest fluorescent quantum yield, the longest lifetime and the lowest cell toxicity. A corresponding mechanism has been proposed. Moreover, as-synthesized N-doped CDs have been applied for selectively detecting the Fe3+ ion and writing letters as a fluorescent ink.

 Please wait while we load your content...
Please wait while we load your content...