Rugae-like FeP nanocrystal assembly on a carbon cloth: an exceptionally efficient and stable cathode for hydrogen evolution†
Abstract
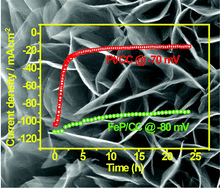
There is a strong demand to replace expensive Pt catalysts with cheap metal sulfides or phosphides for hydrogen generation in water electrolysis. Earth-abundant Fe can be electroplated on carbon cloth (CC) to form high surface area rugae-like FeOOH assembly. Subsequent gas phase phosphidation converts the FeOOH to FeP or FeP2 and the morphology of the crystal assembly is controlled by the phosphidation temperature. FeP prepared at 250 °C presents lower crystallinity and that prepared at higher temperatures of 400 °C and 500 °C possesses higher crystallinity, but lower surface area. The phosphidation at 300 °C produces nanocrystalline FeP and preserves the high-surface area morphology; thus, it exhibits the highest HER efficiency in 0.5 M H2SO4, i.e., the required overpotential to reach 10 and 20 mA cm−2 is 34 and 43 mV, respectively. These values are lowest among the reported non-precious metal phosphides on CC. The Tafel slope for FeP prepared at 300 °C is around 29.2 mV dec−1, which is comparable to that of Pt/CC; this indicates that the hydrogen evolution for our best FeP is limited by the Tafel reaction (same as Pt). Importantly, the FeP/CC catalyst exhibits much better stability in a wide-range working current density (up to 1 V cm−2), suggesting that it is a promising replacement of Pt for HER.

 Please wait while we load your content...
Please wait while we load your content...