MOF-derived ultrafine MnO nanocrystals embedded in a porous carbon matrix as high-performance anodes for lithium-ion batteries†
Abstract
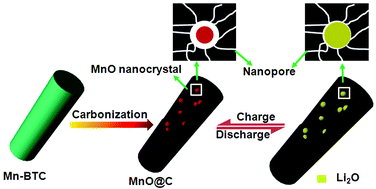
Although MnO has been demonstrated to be a promising anode material for lithium-ion batteries (LIBs) in terms of its high theoretical capacity (755 mA h g−1), comparatively low voltage hysteresis (<0.8 V), low cost, and environmental benignity, the application of MnO as a practical electrode material is still hindered by many obstacles, including poor cycling stability and huge volume expansion during the charge/discharge process. Herein, we report a facile and scalable metal–organic framework-derived route for the in situ fabrication of ultrafine MnO nanocrystals encapsulated in a porous carbon matrix, where nanopores increase active sites to store redox ions and enhance ionic diffusivity to encapsulated MnO nanocrystals. As an anode material for lithium-ion batteries (LIBs), these MnO@C composites exhibited a high reversible specific capacity of 1221 mA h g−1 after 100 cycles at a current density of 100 mA g−1. The excellent electrochemical performance can be attributed to their unique structure with MnO nanocrystals dispersed uniformly inside a porous carbon matrix, which can largely enhance the electrical conductivity and effectively avoid the aggregation of MnO nanocrystals, and relieve the strain caused by the volumetric change during the charge/discharge process. This facile and economical strategy will extend the scope of metal–organic framework-derived synthesis for other materials in energy storage applications.

 Please wait while we load your content...
Please wait while we load your content...