Metallic MoO2 cocatalyst significantly enhances visible-light photocatalytic hydrogen production over MoO2/Zn0.5Cd0.5S heterojunction†
Abstract
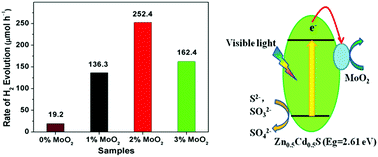
As semiconductor-based nanoheterostructures play a decisive role in current electronics and optoelectronics, the introduction of active heterojunctions can afford new and improved capabilities that will enhance the conversion of solar energy into chemical energy. In this work, a novel metal/semiconductor MoO2/Zn0.5Cd0.5S heterojunction has been designed and prepared to significantly enhance photocatalytic H2 production efficiency. The photocatalytic activity of the as-prepared MoO2/Zn0.5Cd0.5S for H2 generation from water under visible-light irradiation (λ ≥ 420 nm) is measured. MoO2/Zn0.5Cd0.5S hybrid nanoparticles have a higher photocatalytic activity than Zn0.5Cd0.5S even without the noble metal cocatalyst. The results show that the rate of H2 evolution over annealed MoO2/Zn0.5Cd0.5S is about 13 times higher than that of Zn0.5Cd0.5S alone, and 10 times higher than that of simply mixed MoO2/Zn0.5Cd0.5S. Implying that the strong coupling at the interface of MoO2 and Zn0.5Cd0.5S facilitates electron transfer from the conduction band of Zn0.5Cd0.5S to metallic MoO2, thus promoting the separation of photogenerated electrons and holes. MoO2 (2 wt%)/Zn0.5Cd0.5S heterostructured photocatalyst calcined at 673 K achieves the optimal overall activity for H2 evolution. The introduction of metallic MoO2 cocatalyst leads to a remarkable improvement in the photo current and photocatalytic H2 production activity of Zn0.5Cd0.5S, and the content of MoO2 in this catalyst has an important influence on the photocatalytic activity. It is shown that 2 wt% metallic MoO2 loaded on Zn0.5Cd0.5S sample produces a maximum photocatalytic H2 production rate of 252.4 μmol h−1. The junctions formed between metallic MoO2 and semiconductor Zn0.5Cd0.5S by calcination play a key role in high photocatalytic water splitting to produce H2. Our study demonstrates that metallic MoO2 is an excellent H2 evolution cocatalyst, and could be used as a cocatalyst for other semiconductors to improve performances.

 Please wait while we load your content...
Please wait while we load your content...