Carbon-protected bimetallic carbide nanoparticles for a highly efficient alkaline hydrogen evolution reaction†
Abstract
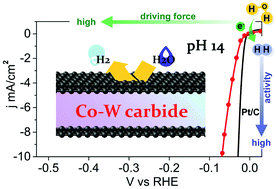
The hydrogen evolution reaction (HER) is one of the two important half reactions in current water-alkali and chlor-alkali electrolyzers. To make this reaction energy-efficient, development of highly active and durable catalytic materials in an alkaline environment is required. Herein we report the synthesis of carbon-coated cobalt–tungsten carbide nanoparticles that have proven to be efficient noble metal-free electrocatalysts for alkaline HER. The catalyst affords a current density of 10 mA cm−2 at a low overpotential of 73 mV, which is close to that (33 mV) required by Pt/C to obtain the same current density. In addition, this catalyst operates stably at large current densities (>30 mA cm−1) for as long as 18 h, and gives nearly 100% Faradaic yield during alkaline HER. The excellent catalytic performance (activity and stability) of this nanocomposite material is attributed to the cooperative effect between nanosized bimetallic carbide and the carbon protection layer outside the metal carbide. The results presented herein offer the exciting possibility of using carbon-armoured metal carbides for an efficient alkaline HER, although pristine metal carbides are not, generally, chemically stable enough under such strong alkaline conditions.

 Please wait while we load your content...
Please wait while we load your content...