Monodisperse SnSb nanocrystals for Li-ion and Na-ion battery anodes: synergy and dissonance between Sn and Sb†
Abstract
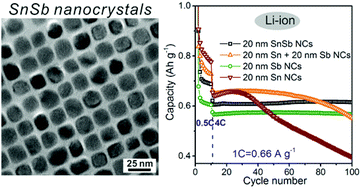
We report a facile chemical synthesis of monodisperse colloidal SnSb nanocrystals (NCs) via reaction between Sn NCs and SbCl3 in oleylamine under reducing conditions. In comparison with individual Sn and Sb NCs and their mixtures, we show that through the creation of SnSb alloyed NCs the Li-ion storage properties are enhanced due to combination of high cycling stability of Sb with higher specific Li-ion storage capacity of Sn. In particular, stable capacities of above 700 and 600 mA h g−1 were obtained after 100 cycles of charging/discharging at 0.5C and 4C rates, respectively (1C corresponding to a current density of 660 mA g−1). Furthermore, Na-ion storage capacities of >350 mA h g−1 and >200 mA h g−1 were obtained at 1C and 20C rates, respectively. This study highlights the differences between Li- and Na-ion (electro)chemistries and the great utility of monodisperse NCs as model systems for understanding size and compositional effects on the performance of conversion-type electrode materials.

 Please wait while we load your content...
Please wait while we load your content...