Serine/threonine ligation for natural cyclic peptide syntheses
Abstract
Covering: 2000 to 2014
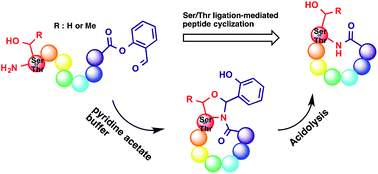
Cyclic peptides are a class of abundant natural products, often exhibiting attractive biological activities. The challenges in the total synthesis of cyclic peptides lie in the preparation of unnatural amino acids if present and the peptide cyclization. Cyclization is an entropy-disfavoured process, with competition between intermolecular and intramolecular reactions. Biological methods can utilize the pre-organized conformation of the side chain unprotected peptide, which brings the reacting termini into proximity, while chemical synthesis requires protecting groups, often large-size and hydrophobic in nature. In this regard, performing peptide cyclization can be an arbitrary and trial-and-error practice. In this highlight, we discuss the application of chemoselective ligation-mediated peptide cyclization in the total synthesis of natural cyclic peptides.

 Please wait while we load your content...
Please wait while we load your content...