Uncovering the structures of modular polyketide synthases
Abstract
Covering: up to 2014
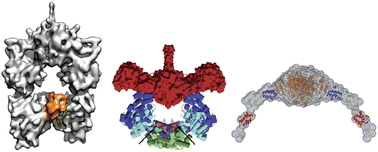
The modular polyketide synthases (PKSs) are multienzyme proteins responsible for the assembly of diverse secondary metabolites of high economic and therapeutic importance. These molecular ‘assembly lines’ consist of repeated functional units called ‘modules’ organized into gigantic polypeptides. For several decades, concerted efforts have been made to understand in detail the structure and function of PKSs in order to facilitate genetic engineering of the systems towards the production of polyketide analogues for evaluation as drug leads. Despite this intense activity, it has not yet been possible to solve the crystal structure of a single module, let alone a multimodular subunit. Nonetheless, on the basis of analysis of the structures of modular fragments and the study of the related multienzyme of animal fatty acid synthase (FAS), several models of modular PKS architecture have been proposed. This year, however, the situation has changed – three modular structures have been characterized, not by X-ray crystallography, but by the complementary methods of single-particle cryo-electron microscopy and small-angle X-ray scattering. This review aims to compare the cryo-EM structures and SAXS-derived structural models, and to interpret them in the context of previously obtained data and existing architectural proposals. The consequences for genetic engineering of the systems will also be discussed, as well as unresolved questions and future directions.
- This article is part of the themed collection: Biosynthetic Assembly Lines

 Please wait while we load your content...
Please wait while we load your content...