Oxidative cyclodehydrogenation of a perylene derivative: different reagents give different products†
Abstract
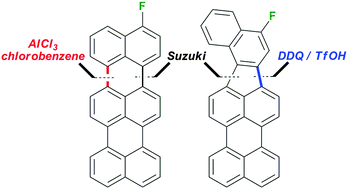
An efficient synthesis of 3-fluoroterrylene, a promising molecular nanoprobe for single electron optical sensing, is described. The key synthetic steps comprised the palladium-catalysed cross-coupling reaction of 3-bromoperylene and 4-fluoronaphthalene-1-boronic acid pinacol ester to give 3-(4-fluoronaphthalen-1-yl)perylene, followed by oxidative cyclodehydrogenation to give selectively either 3-fluoroterrylene or its isomer 10-fluorobenzo[4,5]indeno[1,2,3-cd]perylene. The selectivity of the Scholl oxidation under AlCl3/chlorobenzene or DDQ/TfOH conditions was confirmed by 19F NMR.

 Please wait while we load your content...
Please wait while we load your content...